US Pharm. 2015;40(8):8-11.
As longevity increases, there is greater societal interest in finding lifestyle modifications and treatments for older women to preserve their vitality and quality of life. The urogenital symptoms of menopause are bothersome for patients and negatively affect quality of life. Epidemiologic studies have suggested that estrogen deficiency plays a role in the etiology of lower urinary tract symptoms—70% of women relate the onset of urinary incontinence to their final menstrual period.1 Additionally, urogenital symptoms resulting from postmenopausal estrogen depletion are often confusing for patients with regard to terminology, and are frequently avoided in conversations with healthcare practitioners. Further, concerns about the risks associated with estrogen-progesterone therapy (EPT), such as breast cancer and thromboembolic disease, have caused many patients to avoid the use of this treatment.
Change in Terminology
The urogenital system, alternatively referred to as the genitourinary system, consists of the organs concerned with reproduction and urinary excretion. Although their functions are unrelated, the structures involved in excretion and reproduction are morphologically associated and often use common ducts.2 In 2012, the board of directors of the International Society for the Study of Women’s Sexual Health (ISSWSH) and the board of trustees of The North American Menopause Society (NAMS) formally acknowledged the need to review current terminology associated with genitourinary tract symptoms related to menopause.3 These groups expressed concern that neither of the terms used to describe menopausal symptoms—vulvovaginal atrophy and atrophic vaginitis—were adequate, and they raised the following objections: 1) “vulvovaginal atrophy” describes the appearance of the postmenopausal vulva and vagina; however, it does not describe symptoms; 2) “atrophic vaginitis” implies a pathologic inflammation or infectious process that is not typically present; 3) neither term refers to urinary symptoms.3,4
Therefore, in May 2013, the two societies cosponsored the Vulvovaginal Atrophy Terminology Consensus Conference Panel to discuss a more medically accurate term than vulvovaginal atrophy that is all-encompassing and publicly acceptable, not only for patients but for educators, researchers, and the media. The panel aimed to improve and ease conversations between menopausal women and their healthcare providers.
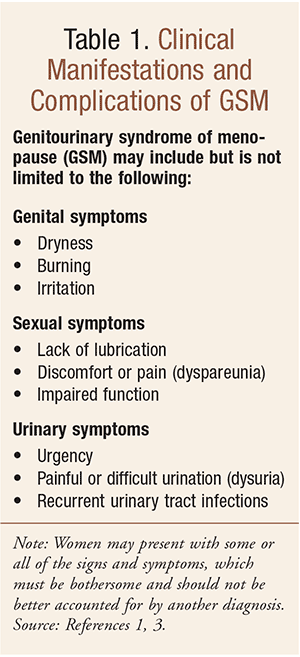
In 2014, the term genitourinary syndrome of menopause (GSM) was presented and discussed at the annual meeting of each society, and the boards of the NAMS and ISSWSH officially endorsed it. The new terminology was introduced by Portman et al in a variety of medical journals including Climacteric, the Journal of Sexual Medicine, Maturitas, and Menopause.3 GSM is defined as a collection of signs and symptoms associated with a decrease in estrogen and other sex steroid hormones involving changes to the labia majora/minora, clitoris, vestibule/introitus, vagina, urethra, and bladder.3 Clinical manifestations and complications of GSM include, but are not limited to, genital symptoms, sexual symptoms, and urinary symptoms (TABLE 1); women may present with some or all of the signs and symptoms, which must be bothersome and should not be better accounted for by another diagnosis.3
Estrogen and Lower Urinary Tract Function
Estrogen is known to have an important role in the function of the lower urinary tract; further, it has been demonstrated that receptors of both estrogen and progesterone are found in the vagina, urethra, bladder and pelvic floor musculature.5,6 Estrogen is involved in a significant way in the continence mechanism such that bladder and urethral function become less efficient with age.7 With regard to urinary continence, estrogens may increase urethral resistance, raise the sensory threshold of the bladder, or increase alpha-adrenoreceptor sensitivity in the urethral smooth muscle.8,9 Postmenopausal-associated estrogen deficiency results in atrophic changes and may be associated with lower urinary tract symptoms such as frequency, urgency, nocturia, urge incontinence, and recurrent infection; these urinary symptoms may also coexist with genital and sexual symptoms as outlined in TABLE 1. Of note, genitourinary prolapse can also cause urinary symptoms, with the prolapse itself causing voiding difficulty; this consideration underscores the importance of a vaginal examination as part of a urinary continence assessment.10
Treatment
In postmenopausal women, studies of treatment with exogenous estrogens have demonstrated beneficial effect with regard to an increase in the number of vaginal intermediate and superficial cells; these changes have been shown to occur in the bladder and urethra as well.11 Importantly, progestogens have been associated with an increase in overactive bladder symptoms and urinary incontinence in those women taking EPT.3
In light of the relationship of estrogen and progestogens to the lower urinary tract and the concerns of women regarding the risks associated with EPT, including breast cancer and thromboembolic disease, first-line therapies for women with symptomatic genitourinary symptoms include nonhormonal lubricants with intercourse and, if indicated, regular use of long-acting vaginal moisturizers. For symptomatic women with moderate to severe genitourinary symptoms and for those with milder genitourinary symptoms who do not respond to lubricants and moisturizers, estrogen therapy either vaginally at low dose or systemically remains the therapeutic standard. The NAMS indicates that when EPT is prescribed, the lowest effective dose for the shortest duration to relieve vasomotor symptoms and genitourinary symptoms is recommended; in women who experience only genitourinary symptoms, low-dose vaginal estrogen is preferred.12,13
Vaginal estrogen, intended to deliver estrogen directly to local tissue, is moderately effective in reducing genitourinary symptoms such as vaginal dryness and burning, difficult or painful sexual intercourse (dyspareunia), urinary frequency and urgency, painful or difficult urination (dysuria), and recurrent urinary tract infections, while minimizing systemic estrogen exposure; even the lowest-dose vaginal ring can successfully reduce urinary frequency and urgency in postmenopausal women.14 Of note, early detection and treatment of vaginal atrophy should be implemented before functional integrity is compromised and irreversible changes occur.15
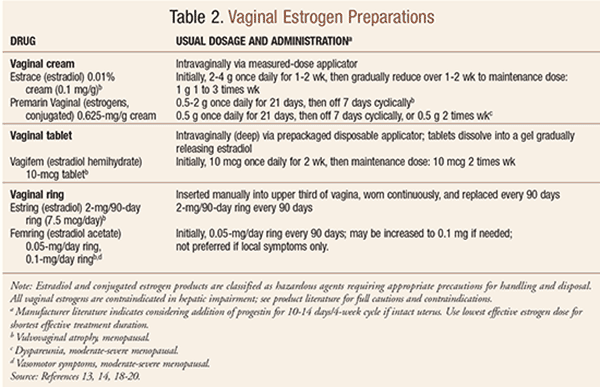
Available vaginal estrogen preparations are listed in TABLE 2 and are categorized as vaginal creams containing estradiol or conjugated estrogens, a low-dose estradiol tablet, and a sustained-release intravaginal estradiol ring. In women with a history of breast cancer and severe vaginal atrophy, vaginal estrogen preparations with the lowest systemic absorption rate may be preferred.16 While it is likely that regular use of low-dose vaginal estrogen will prevent the signs and symptoms of GSM, clinical trial data are available for treatment, not prevention.13 With regard to adverse effects and safety, low-dose vaginal estrogen is considered to have a risk profile that is lower compared with commonly used doses of systemic estrogen therapy owing to its resultant very low serum levels; while available evidence suggests that low doses of vaginal estrogen are generally safe for the endometrium, the long-term data are limited.13 For dyspareunia, OTC nonhormonal vaginal lubricants and moisturizers are highly effective in women for whom vaginal estrogen therapy is contraindicated, for those who wish to avoid hormones, or as a supplement to estrogen; prescription ospemifene is another option for dyspareunia.13,17
Conclusion
Raising patients’ awareness of the vaginal estrogen treatment modalities for symptoms of GSM has the potential to reduce discomfort and pain as well as maintain vitality and quality of life for postmenopausal women who look forward to living through menopause and considerably beyond.
REFERENCES
1. Robinson D, Toozs-Hobson P, Cardozo L. The effect of hormones on the lower urinary tract. Menopause Int. 2013;19(4):155-162.
2. Urogenital system. Encyclopedia Britannica. www.britannica.com/science/urogenital-system. Accessed July 15, 2015.
3. Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. J Sex Med. 2014;11(12):2865-2872.
4. Lewis R. Introducing Genitourinary Syndrome of Menopause. Medscape. Aug 25, 2014. www.medscape.com/viewarticle/830398. Accessed July 14, 2015.
5. Iosif CS, Batra S, Ek A, Astedt B. Estrogen receptors in the human female lower urinary tract. Am J Obstet Gynecol 1981;141:817-820.
6. Batra S, Iosif CS. Progesterone receptors in the female urinary tract. J Urol. 1987;138:1301-1304.
7. Rud T, Anderson KE, Asmussen M, et al. Factors maintaining the urethral pressure in women. Invest Urol. 1980;17:343-347.
8. Versi E, Cardozo LD. Oestrogens and lower urinary tract function. In: Studd JW, Whitehead MI, eds. The Menopause. Oxford, England: Blackwell Scientific Publications; 1988:76-84.
9. Kinn AC, Lindskog M. Estrogens and phenylpropanolamine in combination for stress incontinence in postmenopausal women. Urology. 1988;32:273-280.
10. Wagg A. Urinary incontinence. In: Fillit HM, Rockwood K, Woodhouse K, eds. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. 7th ed. Philadelphia, PA: Saunders Elsevier; 2010:926-938.11. Samsioe G, Jansson I, Mellstrom D, Svanborg A. Occurrence, nature and treatment of urinary incontinence in a 70-year-old female population. Maturitas. 1985;7:335-342.
12. Harvey RA, Champe PC. Pharmacology. 4th ed. Philadelphia, PA. Lippincott Williams & Wilkins; 2009:300-302.
13. The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888-902.
14. Fitzgerald PA. Endocrine disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. 2015 Current Medical Diagnosis & Treatment. 54th ed. New York, NY: McGraw Hill Medical; 2015:1175-1176.
15. Kokot-Kierepa M, Bartuzi A, Kulik-Rechberger B, Rechberger T. Local estrogen therapy--clinical implications--2012 update [in Polish]. Ginekol Pol. 2012;83(10):772-777.
16. Tan O, Bradshaw K, Carr BR. Management of vulvovaginal atrophy-related sexual dysfunction in postmenopausal women: an up-to-date review. Menopause. 2012;19(1):109-117.
17. Reuben DB, Herr KA, Pacala JT, et al. Geriatrics at Your Fingertips. 17th ed. New York, NY: American Geriatrics Society; 2015:296.
18. NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2012. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH). www.cdc.gov/niosh/docs/2012-150/pdfs/2012-150.pdf. Accessed July 14, 2015.
19. Semla TP, Beizer JL, Higbee MD. Geriatric Dosage Handbook. 19th ed. Hudson, OH: Lexicomp; 2014:469-472, 483-485.
20. Epocrates Essentials Version 15.6. Epocrates.com. Updated July 8, 2015. Accessed July 15, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.





